Abstract
Background: Autologous hematopoietic stem cell transplantation (AHSCT) is an important modality in the management of many hematologic malignancies. The first step for patients who are candidates for AHSCT is adequate stem cell collection. Recombinant granulocyte-colony stimulating factor (GCSF) is used for stem cell mobilization. Plerixafor has been used to increase the yield of mobilized stem cells, either upfront or pre-emptively when pre-apheresis peripheral blood CD34+ cell count is low. In the last few years, biosimilar G-CSF (Zarxio®) has become available. Although biosimilar G-CSF may reduce mobilization costs when used alone, the effect on use of additional mobilization agents like plerixafor has not been well studied. Therefore, we investigated whether there was a difference in the rate of Plerixafor usage among patients who were mobilized using biosimilar G-CSF (Zarxio®) compared to the original G-CSF (Neupogen®).
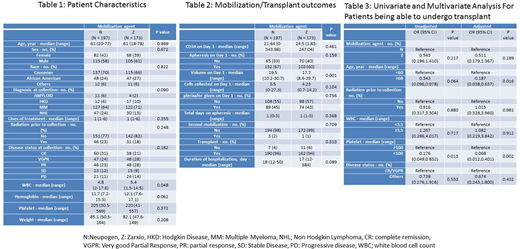
Methods: This was a retrospective single institution study. We utilized the Karmanos Cancer Insitute's blood and marrow transplantation database to collect data on patients who underwent stem cell mobilization for AHSCT using either Neupogen® (N) or Zarxio® (Z), between January 2015 and June 2017. Per institutional policy, patients received G-CSF 10µg/Kg for 5 days and received Plerixafor if peripheral CD34+ cell count on the first planned day of collection was < 20/uL. The target CD34+ stem cell dose for AHSCT was 2 x 106/kg. Kruskal-Wallis tests were used to compare two groups for continuous variables and Chi-squared or Fisher's exact tests for categorical variables. Logistic regression models were used to assess associations between six pre-specified predictors (mobilization, age, radiation therapy, median WBC/ platelet count prior to initiation of mobilization and disease status at time of mobilization) and transplant status.
Results: A total of 370 patients underwent stem cell mobilization during the study period. N was used for mobilization in 197 patients while Z was used in 173 patients. Patient characteristics are shown in Table 1. Median age was 61 years (18-78). There were no significant differences in the baseline patient characteristics other than slightly lower median WBC count prior to mobilization, in patients who received N for mobilization. The indication for stem cell mobilization was Multiple myeloma (67%), Non-Hodgkin's lymphoma (21%), Hodgkin Lymphoma (8%) and Amyloidosis (4%). There was no statistically significant difference in plerixafor utilization between the two groups (45% in N group vs 43% in Z group (p-value: 0.794). There was also no difference between the groups in the peripheral CD34+ cell count on firts planned day of collection, number of collected stem cells, total days of apheresis, need for a second mobilization, rate of transplant and duration of transplant hospitalization (Table 2). In MVA, older age and platelet count < 100 were the only 2 factors which adversely impacted the possibility for a patient to proceed to transplant (Table 3).
Conclusion: There was no difference in plerixafor utilization among the two groups. Previously recognized risk factors like older age and low platelet count were identified as risk factors for not being able to undergo an AHSCT. The cost of biosimilar is about 50% less than the original G-CSF and based on our analysis of a large number of patients at a single institution, biosimilar G-CSF provides significant cost savings for stem cell mobilization.
Deol:Kite Pharmaceuticals: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal